LaBaer Lab | Home
Radiation Biodosimetry
Principal Investigator: Joshua LaBaer, M.D., Ph.D.
Project Manager: Kristin Gillis, MBA
Device Development: Vel Murugan, Ph.D. and Michael Fiacco
Investigators: Mitchell Magee, Ph.D., Jin Park, Ph.D, Garrick Wallstrom Ph.D, and Michael Gaskin
Collaborators: Life Technologies Corporation, Columbia University, and Mayo Clinic
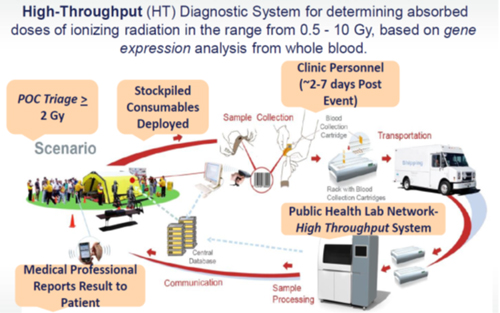
The Biodesign Institute (BDI) at ASU is the lead contractor of a $40M contract awarded in 2009, if all contract options are exercised, that is sponsored by the Biomedical Advanced Research and Development Authority (BARDA). BARDA is responsible for developing a high-throughput (HT) system for radiation biodosimetry. BARDA was established under the Office of the Assistant Secretary for Preparedness and Response (ASPR) to promote innovation and invest in medical countermeasure development that will carry projects through the crucial phases of advanced development to the acquisition of final products. BARDA works within the United States Dept. of Health and Human Services (HHS) Public Health Emergency Medical Countermeasures Enterprise (PHEMCE), to accelerate and facilitate research, development, and acquisition of medical countermeasures for chemical, biological, radiological, and nuclear (CBRN) agents and emerging infectious diseases. BARDA is working to procure biodosimetry assessment tools for rapid determination of an individual’s absorbed dose of ionizing radiation, for screening, triage, and management of clinical facilities, if a radiation incident were to occur.
The key deliverable of this contract is a prototype system capable of processing thousands of blood samples per day for gene expression analysis that will go through at least an US Food and Drug Administration Pre-Submission review needed to conduct clinical studies for a Premarket Submission. This HT biodosimetry system is being designed to provide quantitative, accurate, and reproducible total body absorbed dose measurements of ionizing radiation from 0.5 Gy to 10 Gy (>2Gy differentiation most critical) over a 7- day period.
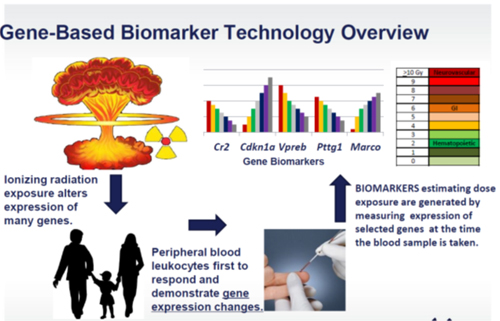
The Center for Personalized Diagnostics (CPD) at BDI is collaborating with Life Technologies Corporation to develop a quantitative, real-time PCR-based platform to rapidly assess an individual's absorption of ionizing radiation. As part of the agreement, researchers from the CPD are designing a multi-gene assay panel that would run on real-time PCR systems from Life Tech such as the Applied Biosystems 7500 Fast Dx and QuantStudio Dx, and would identify individuals exposed to abnormal levels of radiation. Other collaborators include Dr. Sally Amundson of New York's Columbia University Medical Center, who is helping to identify radiation-responsive genes and biodosimetry measurements. The CPD project team uses gene expression discovery techniques such as Agilent microarray and RNA-Seq (core facility) to identify biomarker candidates. Bioinformatics analysis and algorithm development are used to determine the gene biomarkers to quantify absorbed dose. If successful, the Life Technologies team will seek regulatory clearance for the radiation biodosimetry device from the US Food and Drug Administration.

